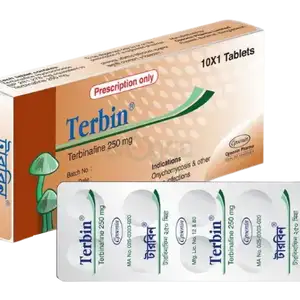
Xfin Tablet
by Square Pharmaceuticals Limited
৳40.00
Opsonin Pharma Limited
Terbinafine Hydrochloride
Terbinafine Purification: This tablet is used to treat nails and nail jogap mycosis caused by dermatophytes (tinea unguium).
Terbinafine Cream: Fungal infections of the skin with Trichophyton (eg, T. rubrum, T. mentagrophytes, T. verrucosum, T. violaceum), Microsporumcanis and Epidermophytonfloccosum. Dermal vaginosis Infection mainly caused by Candida (eg C. arubicans). Catfish wind (catfish wind) by Pityrosporum orbiculare (also known as Marassezia fir fir).
Terbinafine, an allylamine antifungal agent, inhibits the biosynthesis of ergosterol (an essential component of fungal cell membranes) by inhibiting the enzyme squalene epoxidase. This leads to the death of fungal cells primarily due to increased membrane permeability mediated by the accumulation of high concentrations of squalene, but not due to ergosterol deficiency. Depending on the concentration of the drug and the fungal species tested in vitro, terbinafine hydrochloride can kill the fungus. However, the clinical significance of the in vitro data is unknown. Terbinafine has been shown to be active against most strains of the following microorganisms, both in vitro and in clinical infections: Tricophyton Mentagrophyte, Trichophyton Rubrum.
Terbinafine tablet:
Terbinafine granules:
Terbinafine cream: Terbinafine cream can be applied once or twice daily. Cleanse and dry the affected areas thoroughly before application of the terbinafine cream. Apply the cream to the affected skin and the surrounding area in a thin layer and rub in lightly. In the case of intertriginous infections (submammary, interdigital, intergluteal, inguinal) the application may be covered with a gauze strip, especially at night. The likely durations of treatment are as follows:
Relief of the clinical symptoms usually occurs within a few days. Irregular use or premature discontinuation of treatment carries the risk of recurrence. If there are no signs of improvement after two weeks, the diagnosis should be verified.
In vivo studies have shown that terbinafine is an inhibitor of the isoenzyme CYP450 2D6. The drugs mainly metabolized by CYP450 2D6 isoenzymes include the following classes of drugs: tricyclic antidepressants, selective serotonin reuptake inhibitors, β-receptor blockers, class 1C antiarrhythmic drugs (such as fluoro Carney and propafenone) and monoamine oxidase type inhibitors. B. Co-administration of terbinafine should be carefully monitored and the dose of metabolite 2D6 may need to be reduced.
Terbinafine tablets and creams are contraindicated in people allergic to terbinafine.
Reported adverse events include gastrointestinal symptoms (including diarrhea, dyspepsia and stomach pain), abnormal liver tests, rash, hives, itching and dysgeusia. Side effects were generally minor and temporary and did not lead to interruptions. Abnormal reactions based on worldwide experience for the use of terbinafine include: idiopathic and symptomatic liver damage, rarely if it leads to liver failure, death or liver transplantation: Serious skin reaction, severe neutropenia, thrombocytopenia, vascular edema and allergic reaction (anaphylactic). Other abnormal reactions reported include fatigue, fatigue, vomiting, arthralgia, myalgia, and hair loss.
Terbinafine Tablets: There are no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response and treatment of nail fungus may be delayed until late in pregnancy, terbinafine should not be started during pregnancy. pregnant. After oral administration, terbinafine is present in the breast milk of nursing mothers. Treatment with terbinafine is not recommended in nursing mothers.
Terbinafine Cream: Fetal toxicity and fertility studies in animals have not revealed any adverse effects. There is no clinical experience with terbinafine in pregnant women; therefore, unless the potential benefit outweighs the potential risk, terbinafine should not be used. Terbinafine is excreted in human milk and therefore mothers should not be treated with terbinafine while breastfeeding.
Terbinafine tablets: When using terbinafine tablets to treat onychomycosis in people with or without pre-existing liver disease, rare liver failure occurred, some leading to death or liver transplantation. In most liver cases reported related to terbinafine use, patients have serious underlying systemic disease, and the causal relationship with terbinafine is uncertain. In patients with active or chronic liver disease, the severity of the liver event and/or its outcome may be worse. If there is biochemical or clinical evidence of liver injury, terbinafine tablets should be discontinued. Individual cases of severe skin reactions (eg Stevens-Johnson syndrome and toxic epidermal necrolysis) have been reported. If a progressive rash occurs, terbinafine treatment should be discontinued.
Terbinafine Cream: Terbinafine Cream is for external use only. Avoid contact with eyes.
Note: Terbinafine is not recommended for patients with chronic or active liver disease. Before prescribing terbinafine, the pre-existing liver disease should be assessed. Hepatotoxicity may occur in patients with or without pre-existing liver disease. It is recommended that all patients pretreat the testes with serum transaminases (ALT and AST) before taking terbinafine tablets.
Topical antifungal medicines, drugs for subcutaneous and mycoses
Protect from light and store in a cool, dry area below 30°C.
Opsonin Pharma Ltd.
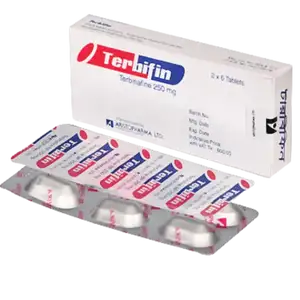
by Aristopharma Ltd.
৳50.00